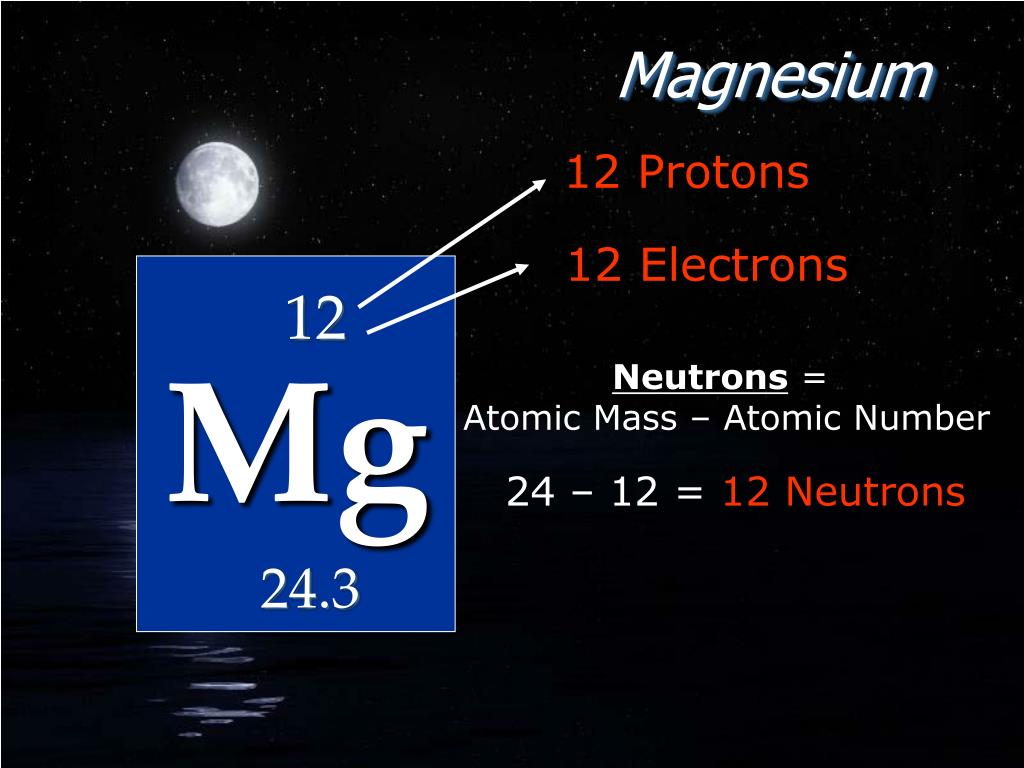
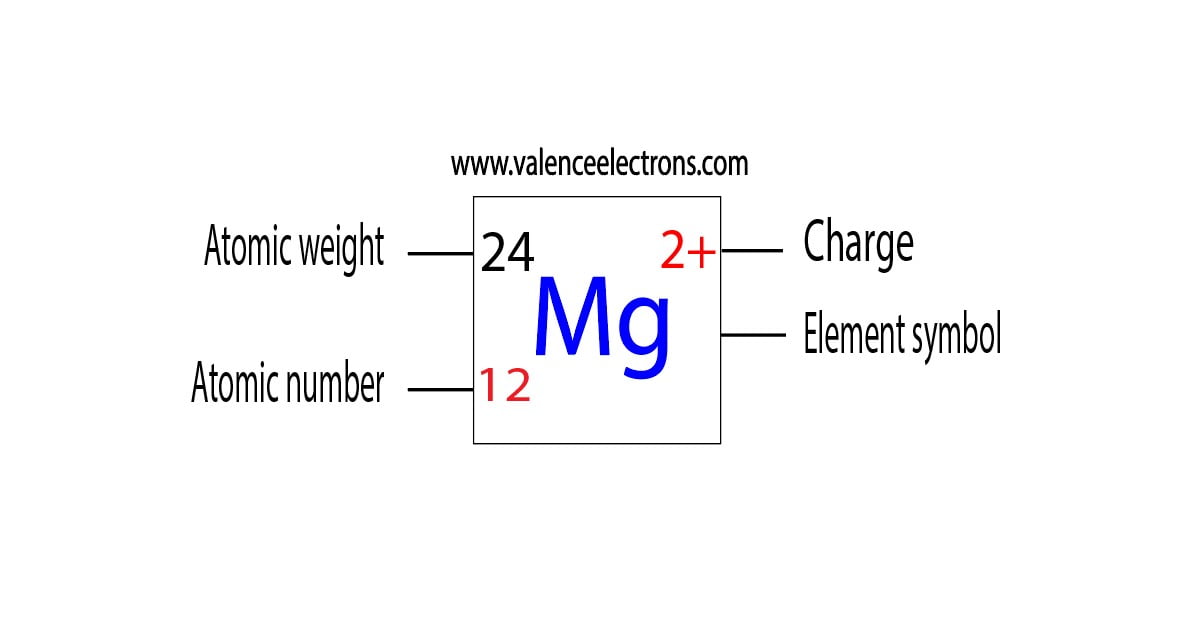
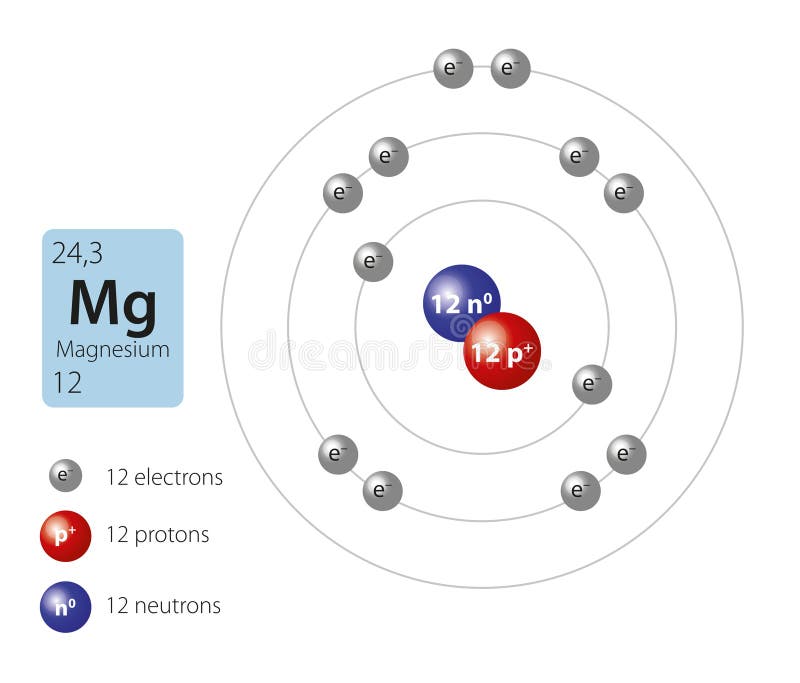
Number of Protons: 12: Number of Neutrons: 12: Number of Electrons: 12: Melting Point: 650.0° C: Boiling Point: 1107.0° C: Density: 2.62 grams per cubic centimeter: Normal Phase:. Magnesium oxide is the byproduct of burning magnesium and can cause respiratory problems like asthma or emphysema. Common Uses: Photography flash products; Bombs;. Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Cite this Article. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element.
PPT Protons, Neutrons, Electrons PowerPoint Presentation, free download ID3287356

Atomic Structure (ALevel) ChemistryStudent

How Many Protons Are In An Atom Of Magnesium? » Doctorsvisions

Magnesium Electron Configuration (Mg) with Orbital Diagram

magnesium electron configuration Newton Desk
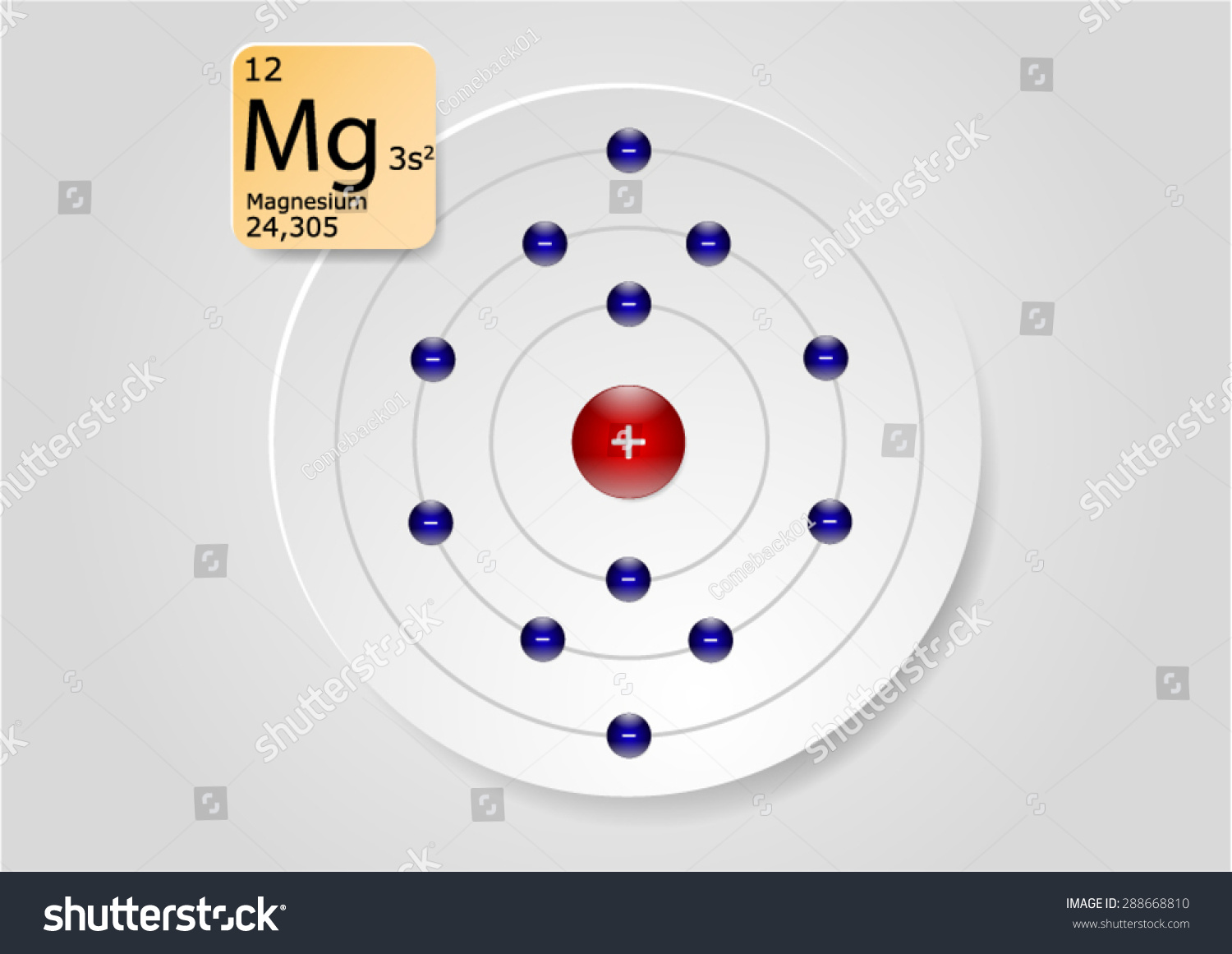
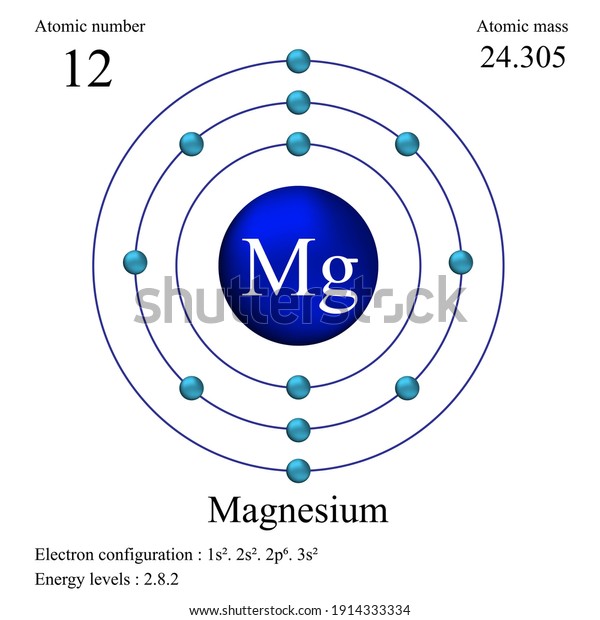
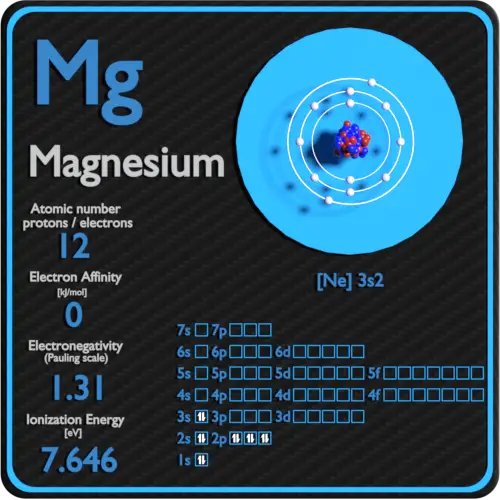
Electron Configuration for Magnesium and ion(Mg2+)

Protons, neutrons and electrons.
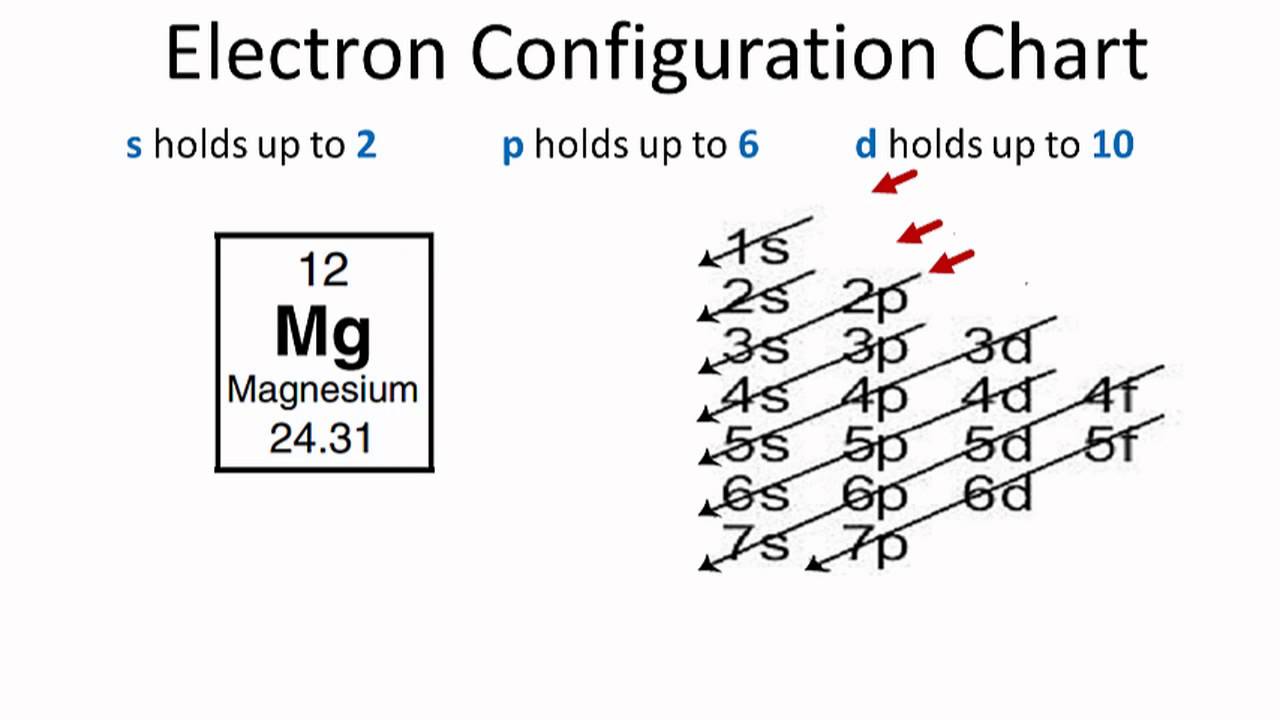
Magnesium Electron Configuration (Mg) with Orbital Diagram
Magnesium Protons Neutrons Electrons Electron Configuration

Magnesium Atom Science Notes and Projects
Model of magnesium atom stock vector. Illustration of mass 164475021
Mg Magnesium Atom Periodic Table Stock Vector 288668810 Shutterstock
La estructura atómica de magnesio tiene vector de stock (libre de regalías) 1914333334
Magnesium Periodic Table and Atomic Properties

FileElectron shell 012 Magnesium.svg Wikimedia Commons Chemistry periodic table, Element
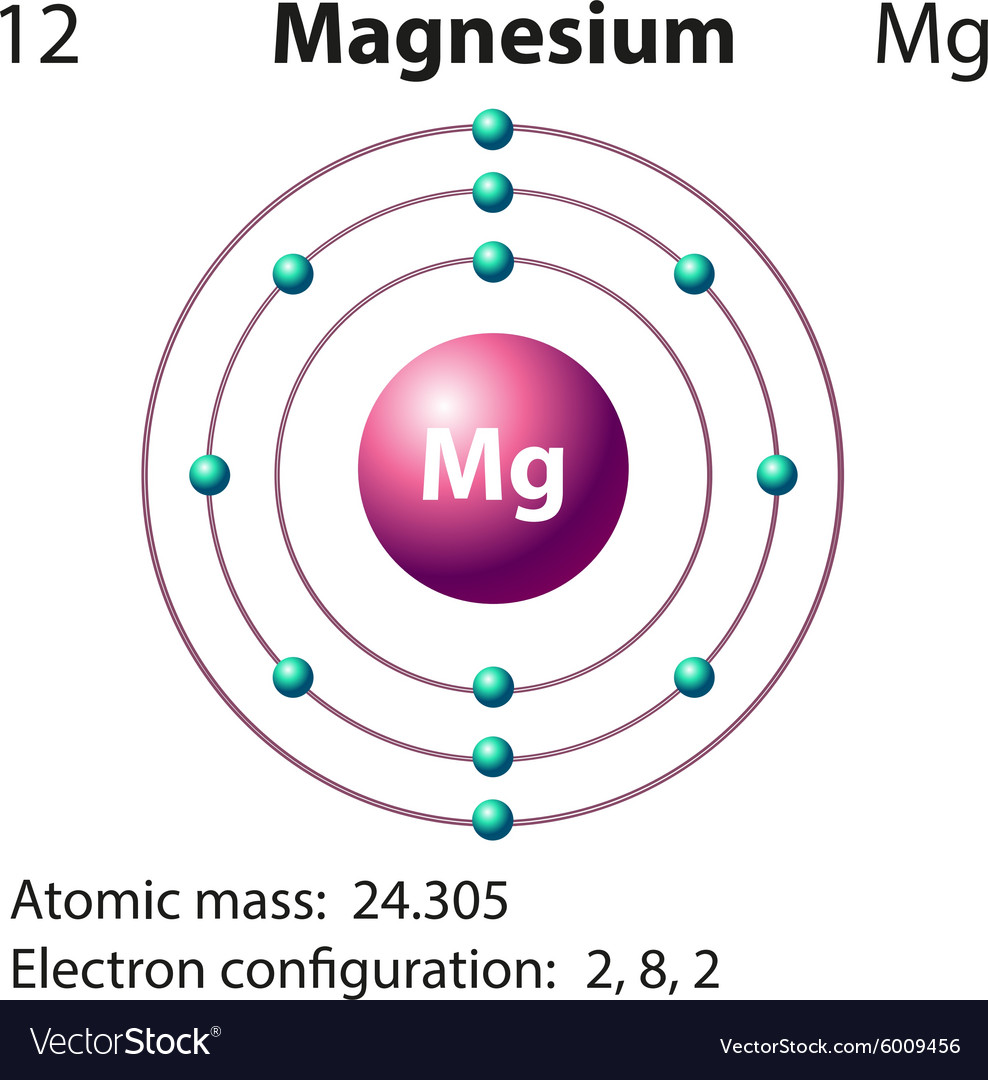
Diagram representation of the element magnesium Vector Image
/GettyImages-1135707671-640473b29d534e15a24491c0d6b2789e.jpg)
Periodic Table Magnesium Electron Configuration Periodic Table Timeline

PPT Nuclear model of atom PowerPoint Presentation ID6309354
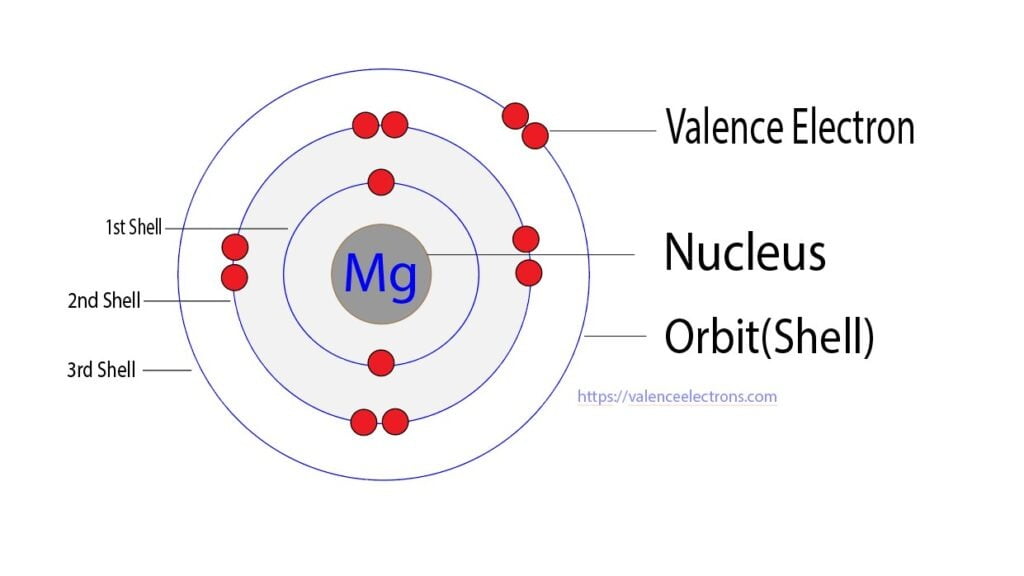
Magnesium(Mg) electron configuration and orbital diagram (2022)
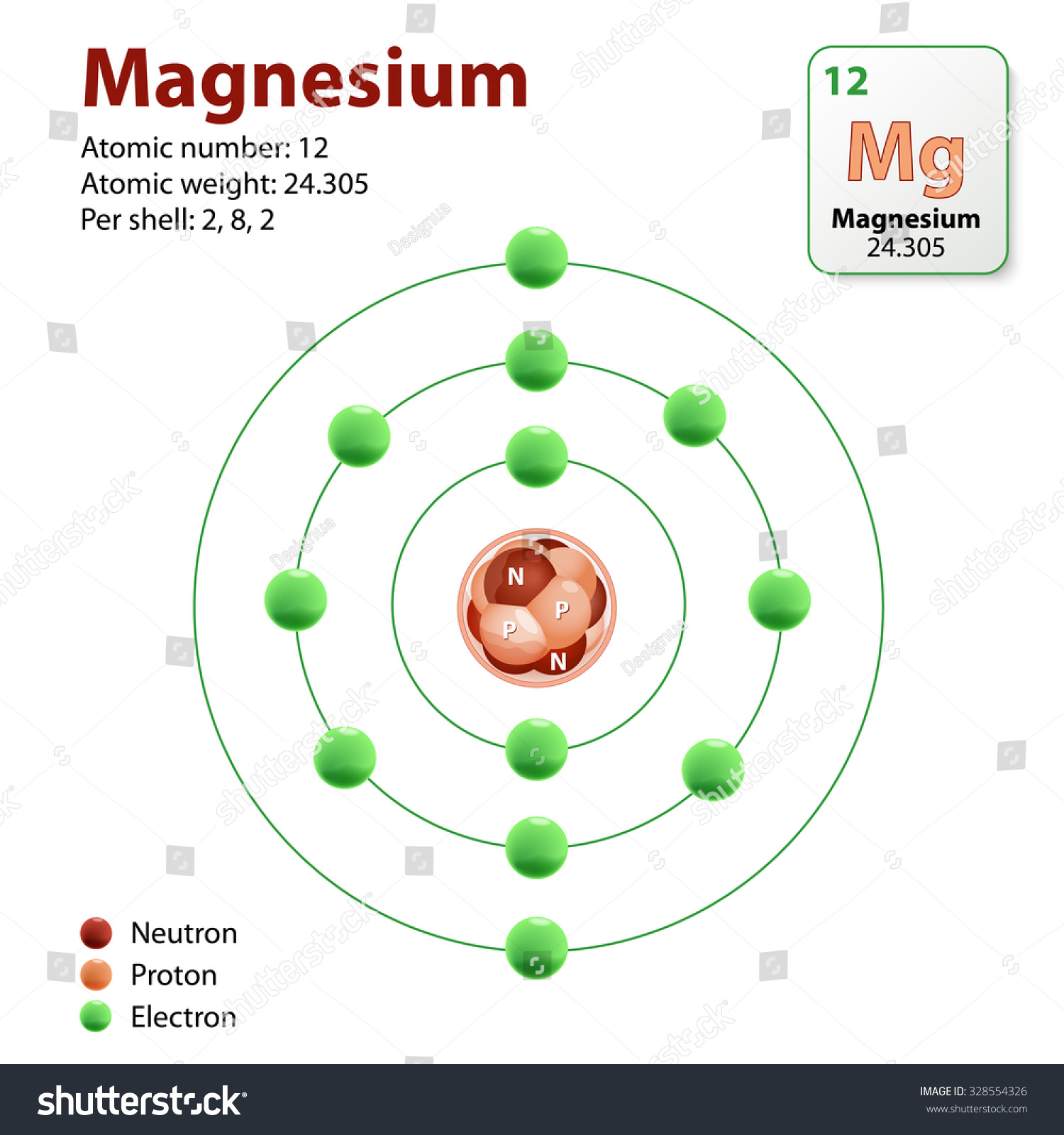
Diagram Representation Element Magnesium Neutrons Protons Stock Vector 328554326 Shutterstock
Thus, the number of electrons in Magnesium is 12. Summary. Number of Protons in Magnesium. The number of protons can be found by knowing the atomic number of that atom. Number of Protons in Magnesium = Atomic number of Magnesium = 12; Number of Neutrons in Magnesium. The number of neutrons can be found by subtracting the atomic number from its.. Make sure that you round the atomic mass to the nearest whole number. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11. 6. Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass.